Abstract
Background: Cancer immunotherapy, and checkpoint inhibition in particular, is poised to radically transform our approach to treating cancer. Recent clinical successes with checkpoint inhibitors and immuno-oncology agents have demonstrated the importance of the immune system in controlling and treating cancers. Early indications that tumor cells can generate an antitumor immune response were evidenced by the varying degrees of immune cell infiltration found in the tumor microenvironment (TME). Despite the presence of this immune infiltrate, it has long been suspected that immune function is defective or inhibited by the TME. The rapid series of new drug approvals in diverse tumors ranging from melanoma, kidney, lung, bladder and Hodgkin lymphoma show that the anti-tumor immune responses can be activated for clinical benefit, however, our understanding of the mechanics of immune response to immuno-oncology agents has lagged behind the clinical successes. Furthermore, responses to immuno-oncology agents are not universal and mechanisms of resistance are poorly described. In large part, this is due to limitations of currently available technology which does not allow for detection of complex immunophenotypes in tissue sections. High parameter methods such as gene expression profiling or flow cytometry have been applied to study the TME, however data on cellular heterogeneity and rare cells is lost with gene expression studies and the spatial relationship between tumor and immune cells is lost with flow cytometry. The Fluidigm Hyperion imaging mass cytometry (IMC) system combines a CyTOF mass cytometer with a laser ablation system allowing for 40+ parameter simultaneous immunophenotyping on a single slide of FFPE tissue, with sub-cellular resolution. Here we describe our efforts to develop and optimize highly multiplexed assays for the characterization of immune cells in the TME in various tumors on the IMC platform.
Methods: Literature was reviewed to identify markers relevant to TME. Metal conjugated antibodies were obtained from Fluidigm, and supplemented with custom conjugations where required. Antibodies were validated on FFPE tissue sections of lymphoma, colon cancer, and normal lymph node as controls. Antigen retrieval, blocking, and antibody staining concentrations were optimized to identify universal staining conditions. Membrane and nuclear/DNA staining was performed to aid in cell segmentation. Slides were imaged using the Fluidigm Hyperion IMC system with a 1µM laser ablation spot size and frequency of 100-200 Hz. Area of analyzed tissue varied from 400 um2 up to 1000 um2 with multiple areas imaged per specimen. Image analysis was performed with MCD viewer (Fluidigm) or CellProfiler/miCAT (https://doi.org/10.1101/109207).
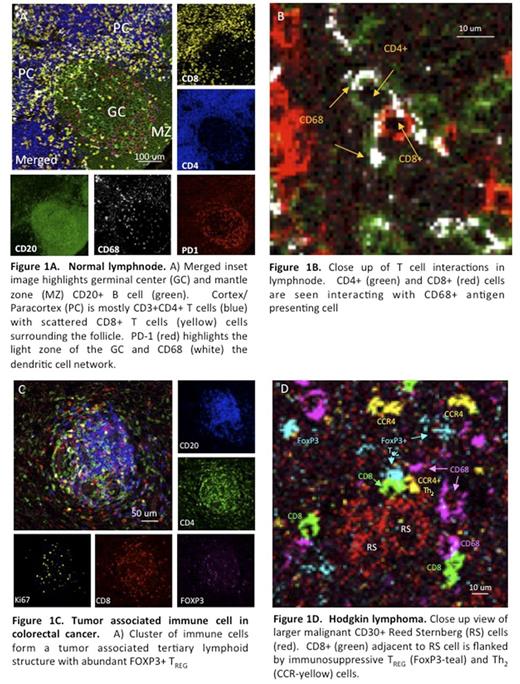
Results: We successfully performed IMC imaging of normal lymph nodes (LN), colon cancer, Hodgkin lymphoma, NK lymphoma by using a 26 parameter panel. We were able to clearly identify tissue architecture in the normal lymph node, germinal center (GC), mantle zone(MZ), cortex/paracortex(PC) (Fig. 1A), and with enough resolution to observe three way interaction between CD4+ T cell, CD8+ T cell and CD68+ antigen presenting cell (Fig. 1B). In tumors we were able to characterize specific populations such as CD8+Tcells, Th1/Th2 subsets, FoxP3+Treg, tissue associated macrophages, CD56+NK cells and tumor vasculature. Important immuno-oncology targets such as LAG3, PDL1/PDL2 and PD1 were measured. Tertiary lymphoid structures in colon cancer were found to have abundant FoxP3+Treg (Fig. 1C). In Hodgkin lymphoma we were able to observe tumor [Reed Sternberg (RS) cells], effector and regulatory cell interactions at a single cell level. CD8+ cells (green) adjacent to RS cell are flanked by immunosuppressive TREG (FoxP3-teal) and Th2 (CCR-yellow) cells (Fig. 1D).
Conclusion: IMC allows for successful multiplex imagining of the TME in FFPE tissues. The method does not require special processing and can be applied to archival specimens and tumor microarrays. Methods developed here should be applicable for the study of the TME in other tumor types and could be used to identify additional biomarkers of response to immuno-oncology agents.
Merchant: Fluidigm, Inc: Research Funding; Pfizer, Inc: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal